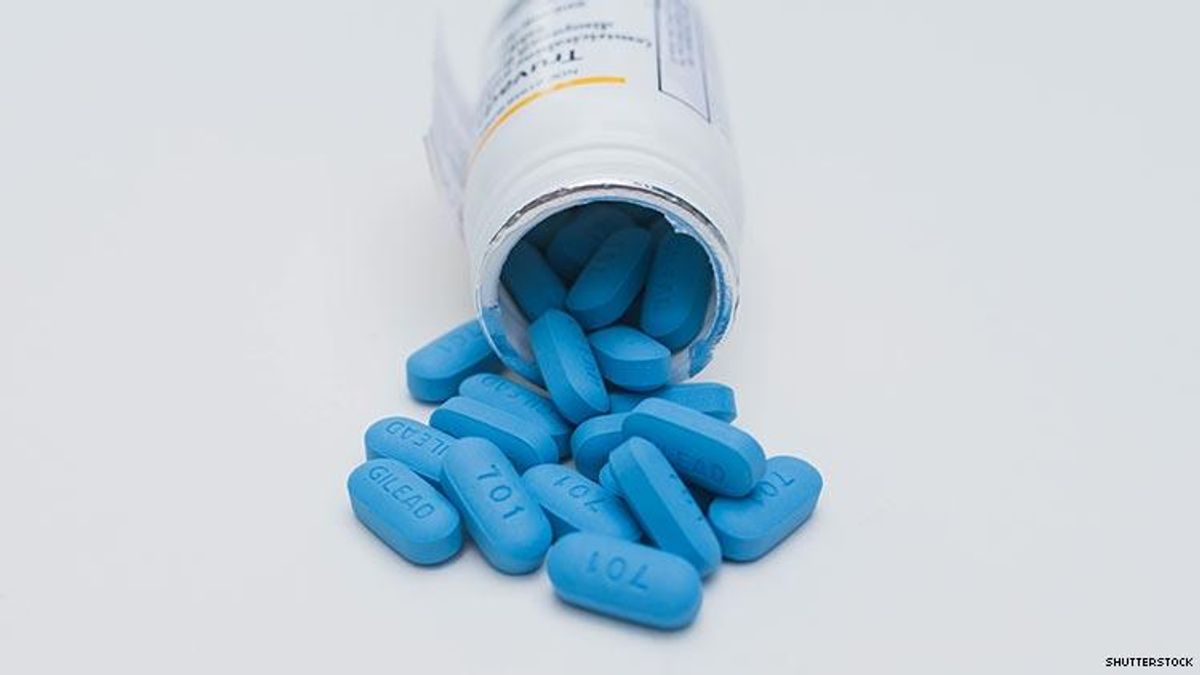
The Department of Health and Human Services is suing the maker of Truvada and Descovy for patent infringement.
The legal move from President Donald Trump's administration comes after the pharmaceutical company Gilead Sciences has made billions from pre-exposure prophylaxis, or PrEP, a daily HIV prevention regimen that when taken properly makes it virtually impossible to contract the virus. Truvada and Descovy are the only drugs approved for use as PrEP.
"HHS recognizes Gilead's role in selling Truvada and Descovy to patients for prevention of HIV. Communities have put these drugs to use in saving lives and reducing the spread of HIV," HHS Secretary Alex M. Azar II said Wednesday in a press release.
"However, Gilead must respect the U.S. patent system, the groundbreaking work by CDC researchers, and the substantial taxpayer contributions to the development of these drugs," Azar said.
The unusual step comes after the drug giant used a system researched and discovered by the Centers for Disease Control and Prevention to find that a medication already on the market for HIV treatment could be used for prevention.
Gilead has come under heavy fire regarding the pricing of Truvada.
"Gilead Sciences has inflated the cost from $6 to more than $1,600 per month, despite the US taxpayer paying for almost the full cost of its development. If we could lower the price of the drug, we could end the HIV epidemic without a vaccine," reads a statement from the group Break the Patent.
Gilead did announce earlier this year it would allow competitor Teva to make a generic version of Truvada.
Months later, the Food and Drug Administration authorized Gilead to bring Descovy to the market. Gilead scientists say Descovy "was shown in clinical trials to be less toxic than Truvada to the kidneys and the bones."
HHS notes Gilead originally obtained FDA approval for the use of Truvada and Descovy products for HIV treatment in combination with other drugs. But it was CDC researchers, including costly human trials, that developed the PrEP regimens. HHS holds a patent on that science, but Gilead has "repeatedly refused to obtain licenses for the use of the HHS patents," reads an agency press release.
The PrEP process was first discovered by CDC scientists in the mid-2000s and confirmed through human trials costing hundreds of millions of taxpayer dollars.
HHS holds four patents protecting the CDC's work. Two companies besides Gilead have agreed to pay licensing fees to HHS to produce generic equivalents to Truvada for PrEP in other nations.












































































Fans thirsting over Chris Colfer's sexy new muscles for Coachella